 |
Roger
J.W. Truscott and John T. Tholen, Bio-organic
Chemistry Research Unit, University of Wollongong N.S.W.
Summary
A method for the separation and quantification of glucosinolates
from plant tissue Is described.
Following enzymic desulfation, the corresponding desulfoglucosinolates
are separated using reversed phase HPLC in less than 30 minutes.
Accurate quantification of each glucosinolate can be obtained
using this method.
|
 |
Introduction
Glucosinolates are a group of secondary metabolites found
in all members of the plant family Cruciferae. This family
contains many representatives of economic importance to man
including cabbage, cauliflower, broccoli, brussels sprouts,
swede and mustard. The flavour of these species is to a large
part governed by the content of glucosinolates present. These
chemicals are also of interest since they act to protect the
plants which contain them from attack by pathogens such as
fungi and bacteria. At higher levels however, such as those
found in oil seeds (e.g: rapeseed), glucosinolates and their
breakdown products can have adverse effects on the growth
rates of non, ruminant animals when the seed meal is used
in the diet (1).
For this reason plant breeders are attempting to decrease
the levels of glucosinolates in rape and mustard seeds.
Several
methods have been used for monitoring glucosinotate Levels.
These include gas chromatography of the isothiocyanate breakdown
products resulting from myrosinase digestion of the glucosinolates
and gas chromatography of the silyl derivatives of intact
gluco- sinolates. There are a number of problems associated
with the use of these G.C techniques, for example neither
can measure the content of the indole gucosinolates.
For
this reason HPLC has been developed fbr the analysis of glucosinolates
in plant tissues. The procedure employs on-column enzymic
desulfation of extracted glucosinolates followed by HPLC separation
of the resulting desulfoglucosinolates (2). |
 |
HPLC
A powerful tool
for separating
and quantifying
complex plant
metabolites
|
| |
Materials
and Methods
Instrumentation
ICl/Knauer HPLC Pump 64(2) ICl/Knauer Gradient Programmer
50B ICl/Knauer Variable Wavelength UV- Vis Detector Model
87
ICI 5 micron ODS 2 SphensorbT" HPLC Column (4.6mm x 250mm)
Chemicals
HPLC grade water
HPLC grade Acetonitrile -Mallinckrodt Plant Material:
Rapeseed (Brassica napes)
Brussels Sprouts (Brassica oleracea)
Chromatography Conditions
A binary gradient of Water (A) and 20% Acetonitrile (B) was
used.
Gradient Programme |
|
Results
and Discussion
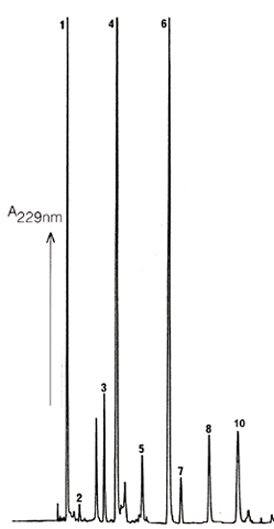
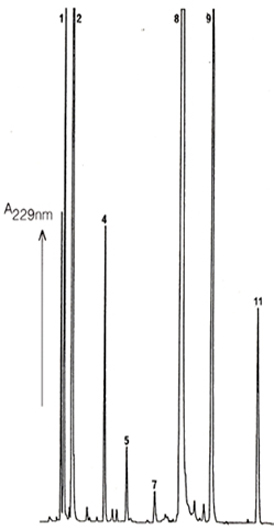
Excellent resolution of the different glucosinolates as desulfoglucosinolates
can be obtained by Reversed Phase HPLC using ICI Spherisorb
ODS2 columns. This is true for the glucosinolates found in seed
tissue (Fig. 1) and also those extracted from leaf (Fig. 2).
The identities of the glucosinolates in the numbered peaks is
given in Table 1 This HPLC method does not use buffer salts
so it is a simple matter to increase the amount of material
injected to allow collection of the individual desulfo-glucosinolates.
These can then be used for structure determination, for example
by derivatization and gas chromatography/mass spectrometry,
if new or unusual glucosinolates are suspected. This approach
was used in the identification of 4-hydroxyindole glucosinolates
(4) and 4-methoxyindole glucosinolates (5). |
|
Alternatively
this technique can be used in order to collect sufficient
of each desulfoglucosinolate for the preparation of standard
curves versus an internal standard such as o-nitrophenyl galactoside
(3) Thus all glucosinolates can be quantified with minimal
detectable limits of the order of 30 ng.
These are not the only advantages which the HPLC method enjoys
over the previously used GC methods( 6) .
1. No derivatisation of the desulfoglucosinolatesis necessary
and the eluate arising from on-column arylsulptase digestion
can be injected directly onto the HPLC.
2.
The four indole glucosinolates (3-indolylmethyl-GS, 4-hydroxy-3-indotylmethyl-GS,
4-methoxy-3-indoylmethyl-GS, and 1-methoxy-3-indolytmethyl-GS)
are well resolved. |
| |
Time
Mins |
Flow
mL/min |
%A |
%B |
0 |
1.5 |
100 |
0 |
1.00 |
1.5 |
100 |
0 |
21.00 |
1.5 |
0 |
100 |
26.00 |
1.5 |
0 |
100 |
30.00 |
1.5 |
100 |
0 |
Detector:
229 nm
Sample
Preparation Summary
1.
Seed or seed meal is extracted with boiling water. Vegetative
tissue is extracted with boiling methanol.
2. Proteins are precipitated
3. Supernatent is applied to DEAE Sephadex A-25
4. Positively charged and neutral species are removed by washing
with water.
5. Aryl sulfatase is added to the column.
6. Desulfoglucosinolates are eluted.
7. Internal standard can be added.
8. An aliquot is injected directly onto the HPLC.
The method is described in detail in reference 3. |
|
 |
|
 |
| |
|
|
Figure
1 Seed
Reversed Phase HPLC Chromatogram of Desulfoglucosinolates obtained
from rapeseed, Cultivar "Bunyip" |
|
Figure
2 Leaf
Reversed Phase HPLC Chromatogram of Desulfoglucosinolates obtained
from Brussels Sprout Leaf. |
| |
|
|
|
|
|
|
| |
|
|
|
|
|
| |
3.
Both indole and non-indole gluco-- sinolates form desulfogluco-
sinolates and thus the relative amounts of each can be compared
directly in the one chromatogram.
4. No degradation of gluco-sinolates has been observed by
HPLC.
The sulphoxide containing desulfo-glucosinolates chromatograph
as single peaks as do the indole glucosinolates. |
|
In
addition, the rapid and simple isolation and analytical methods
ensure minimal degradation of labile glucosinolates such as
4-hydroxy-3-indolylmethyl-GS.
5.
All of the major glucosinolates encountered so far in the
various plant species are well resolved and there seems to
be little or no interference from nonglucosinolate substances. |
|
|
| |
 |
|
Conclusion
This example of the analysis of glucosinolates in plant tissues
serves to illustrate the power of conventional gradient HPLC
equipment for the separation and quantification of biological
metabolites. Similar HPLC techniques are being employed for
the analysis of other important plant chemicals.
The HPLC method described in this application note offers
a great number of advantages over previously used GC methods
and thus it is not surprising that it is currently being adopted
worldwide for the analysis of glucosinolates in plants.
|
|
References
1 Fenwick. G.R.;,Heaney., R.K.; and Mullin,
W.J.
Critical Reviews in Food Science and Nutrition (1983) 18, 123-200.
2 Truscott, R.J.W.; Minchinton, l.R.; and Sang,
J.P.
J. Sci. Food Agri. (1983) 34, 247-254.
3 Sang, JP.; and Truscott, R:J.W.
J. Assoc. of Anal. Chem. (1984) 67, 829-833.
4 Truscott,-R.J.W.; Burke, D.G.;,and Minchinton,
I.R
Biochem. Biophys. Res. Commun. (1982a)107, 1258-1264..
5 Truscott, R.J.W.; Burke, D.G.; and Minchinton,
Biochem. Biophys. Res. Commun. (1982b) 107, 1368-1375.
6 Sang, J.P.; Minchinton, l.R.; Johnston, P.K.;
and Truscott, R.J.W. Can. J. Plant Sci. (1984)'64, 77-93 |
